Endomag has received an extended indication for the long-term use of the Magseed® magnetic marker in any soft tissue. The extended indication was approved after the Magseed® marker was found to be accurate and easy to place in the breast and the armpit in a number of clinical studies. CE certification widening follows the 510(k) clearance from the U.S. Food and Drug Administration (FDA), which was received for the same indication already in 2018.
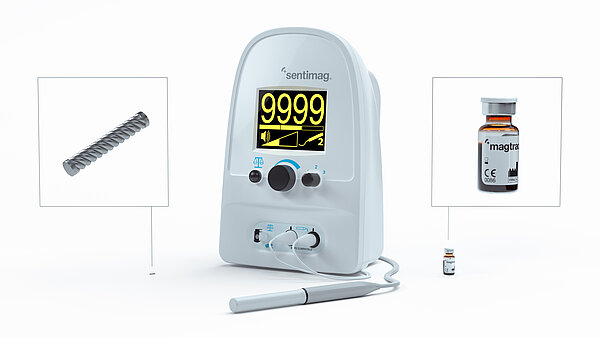
Magseed® is distributed throughout EMEA by Sysmex as part of the Sentimag® surgical guidance platform.
The Magseed® marker is a tiny seed made from surgical grade steel. It is placed in tissue to mark tumours or lymph nodes before surgery to help the surgeon accurately locate and remove the cancer sites in the operating room. It is very robust, and once placed, stays firmly in place1 and is detected by the Sentimag® probe with precision.
The Magseed® marker has already enabled tens of thousands of women to benefit from the more accurate marking and removal of breast tumours. It now becomes the first tissue localisation marker in Europe that physicians can place days, weeks or even months ahead of surgery to mark any cancer site for accurate removal. Results from two independent studies conducted by researchers from the MD Anderson Cancer Center and the University of California, San Francisco, demonstrated the ease and accuracy of marking positive lymph nodes with a Magseed® and 100% retrieval success.2,3
Nowadays, more women are receiving neoadjuvant systemic therapy (NAST) before surgery to help shrink their tumour and the suspicious lymph node/s. However, treating the tumour so early makes it difficult to determine if the cancer has spread beyond the breast when the patient comes to surgery. Through NAST, lymph nodes tend to shrink and changes to the lymphatic drainage pathway might occur so that a formerly suspicious node might not be clearly identifiable anymore after treatment.
With the new indication, the Magseed® marker can now be used to mark suspicious lymph nodes before or after chemotherapy. Together with Endomag’s lymphatic mapping agent Magtrace®, it allows the surgeon to perform a more targeted dissection to increase the accuracy of the surgery and determine if the cancer has spread. This paired procedure of a sentinel lymph node biopsy (SLNB) and the removal of the previously marked node is called targeted axillary dissection (TAD). Research suggests that TAD makes the staging more accurate compared to SLNB only after NAST.4 Sentimag® is the only wire-free, non-radioactive system for TAD in one platform on the market.
“When it comes to marking lymph nodes in patients receiving neo-adjuvant chemotherapy there are very few options – wires are notoriously difficult to place and can easily migrate often resulting in multiple lymph nodes being removed but not the one you necessarily wanted to take. It’s a huge step forward that we can now use the Magseed marker to mark lymph nodes across Europe, which will allow us to offer our patients more targeted surgery and accurate staging.” Mr James Harvey, Consultant Oncoplastic Breast Surgeon, University Hospital South Manchester.
For further information visit: www.sysmex-europe.com/magseed
About Endomag
Endomag is a global technology company that believes everyone deserves a better standard of cancer care. Many of the world’s leading physicians and hospitals use the company’s technologies to help women with breast cancer avoid surgery when it isn’t needed, and experience better outcomes when it is. To date, the company has helped tens of thousands of women around the world access more precise and less invasive breast cancer care. To learn more visit: www.endomag.com
About Sysmex Europe GmbH
Sysmex aims to shape the advancement of healthcare by providing healthcare professionals around the world with a broad range of medical diagnostics products and solutions. Our total solutions combine highly dependable, multi-functional and easy-to-operate instruments, a variety of reagents, software that can consolidate testing data, and reliable service and support. Sysmex Europe GmbH located near Hamburg, Germany, is a subsidiary of the Sysmex Corporation in Kobe, Japan. From our German offices, we serve our affiliates, distributors and customers throughout EMEA. For more information on how we are lighting the way with diagnostics, visit www.sysmex-europe.com.
References
1 Harvey, JR, et al. (2018). Safety and Feasibility of Breast Lesion Localization Using Magnetic Seeds (Magseed): a Multi-Centre, open-label cohort study. Breast Cancer Res Treat. 169(3):531-536.
2 Greenwood 2019 - Feasibility of Magnetic Seeds for Preoperative Localization of Axillary Lymph Nodes in Breast Cancer Treatment. doi.org/10.2214/AJR.19.21378.
3 Simons 2019 – Prospective Trial of Magnetic Seed Localization of Clipped Nodes after Neoadjuvant Chemotherapy in Node Positive Breast Cancer. MD Anderson Cancer Center. ASBrS 2019, Dallas.
4 Caudle et al. (2016) - Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection.: Journal of Clinical Oncology. 34(10):1072–8.